On December 30, the International Union of Pure and Applied Chemistry announced that elements 113, 115, 117 and 118 are now approved to join the prestigious periodic table. Their addition completes the group of man-made elements that make up the table's 7th row, and brings the total number that will need to be memorized by students to 118!
For those of you that do not know or cannot recall, the periodic table is a tabular list of chemical elements organized by the number of protons that each has in its atomic nucleus. For example, hydrogen is element number 1 because it has one proton, helium is number 2 because it has two and so on and so forth
The first table that was created in 1869 by Russian chemist Dmitri Mendeleev listed 59 naturally occurring elements, with room for 33 more. However, even after the last natural element uranium that has an astounding 92 protons had been found, scientists were not happy.
Based on the characteristics of the different elements they knew there were others just waiting to be discovered. Researchers hypothesized that the reason we are unable to find naturally occurring examples is because the elements are extremely heavy and, therefore, decay quickly.
Hence began the race to synthesize elements in the laboratory. This, of course, is no easy task. To create the chemical structures researchers have to slam an existing element with the ions of another in hopes that they will fuse to form a new element. The chances of this happening are of course slim to none.
Even those that are successful are highly volatile. This means a newly created element could disappear in the blink of an eye. Of the most recent ones, 113 has the longest half-life of 19.6 seconds, while 118, which was created by slamming californium with calcium ions has the shortest, a mere 0.89 milliseconds.
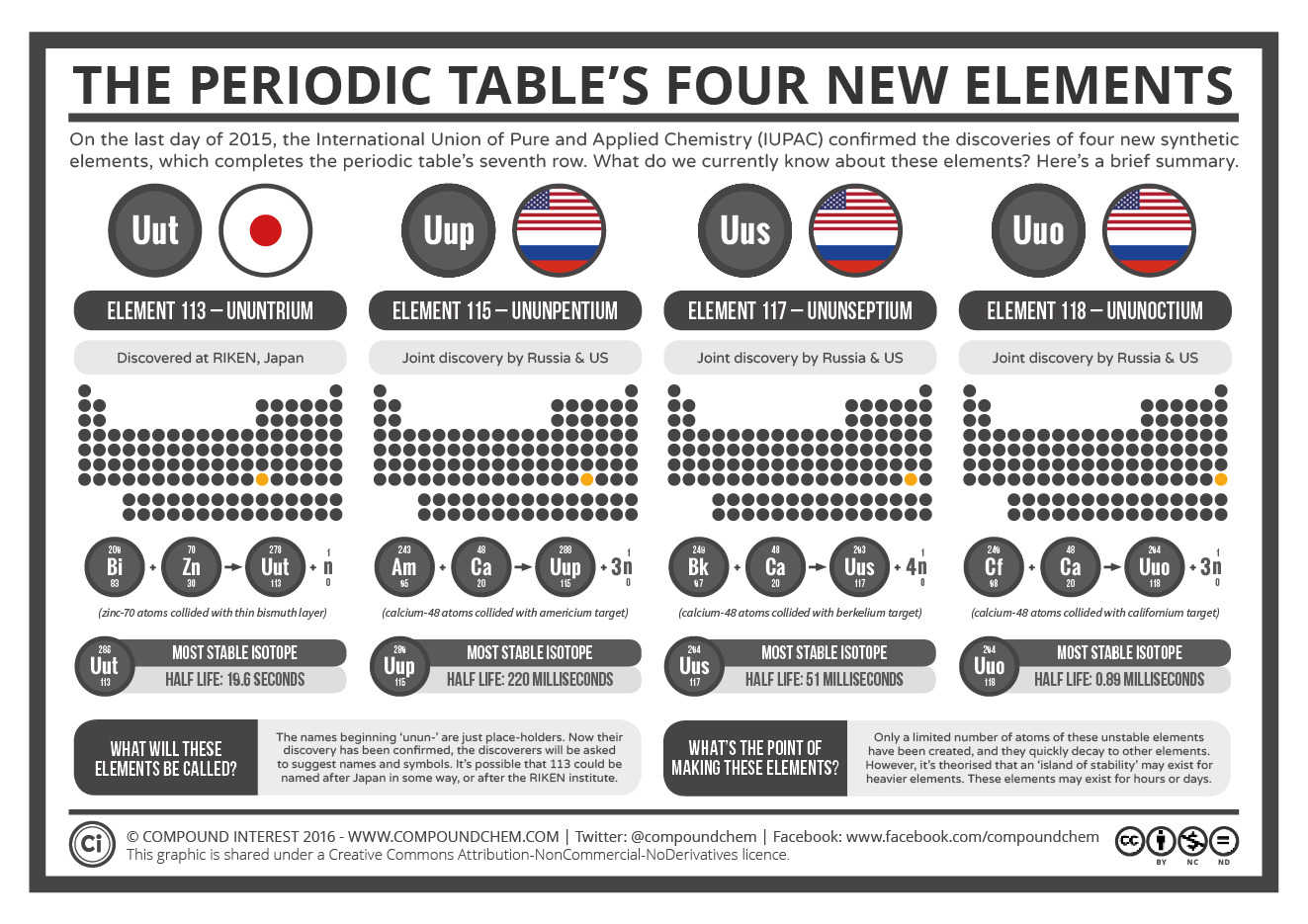
While that is difficult enough, being approved by the International Union of Pure and Applied Chemistry is even more so. That's because to be considered for the periodic table the new element has to be reproduced by another scientist working in a non-affiliated laboratory. In many cases, this can take years! Element 113, for example, was first synthesized in 2003, while the newest - 117, was created five years ago.
So why do scientists go through years of research to create elements that have little practical use? Believe it or not, it is not to make your life harder, but mainly to challenge themselves. There is also an ongoing race between scientists, to see who can create the most new elements. Given that three of the four recently added ones were first manufactured in the United States do not be surprised if another team tries to up the ante with an eighth row!
Resources: qz.com, fastcompany.com,gizmag.com,riken.jp.com